‡ indicates equal contribution; * indicates corresponding authors
15. Robinson J. D. and O’Flaherty D.K.* “Nonenzymatic primer extension experiments in the presence of coacervates”. Current Protocols in Nucleic Acid Chemistry, 2025, accepted
14. Saraya J. S. and O’Flaherty D. K.* “Derivatization of solid-supports using a carbamate linked tether containing a disulfide functional group”. Current Protocols in Nucleic Acid Chemistry, 2025, accepted
13. Saraya J. S. and O’Flaherty D. K.* “An aqueous-compatible on-column approach for the conjugation of nucleic acids using amino modifiers”. Current Protocols in Nucleic Acid Chemistry, 2025, accepted
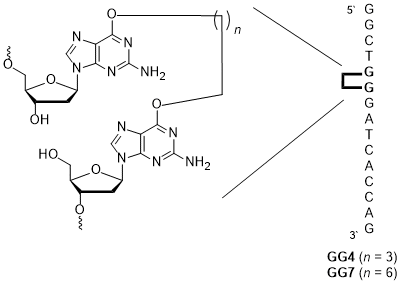
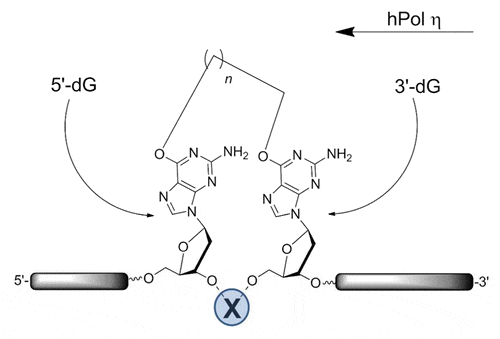
12. Capperauld M. J.‡, Valluru K. K.‡, Saraya J. S., Zakaria E., Wong C. E., O’Flaherty D. K.* “Simple diaminonucleoside-mediated nonenzymatic ligation of oligonucleotides”. Bioconjugate Chemistry, 2025, DOI:10.1021/acs.bioconjchem.5c00090.

11. Saraya J. S., Horton N. G., Capperauld M. J., Zakaria E., O’Flaherty D. K.* “A Versatile Disulfide-containing Solid-support Strategy for 3′-modifiers in Oligonucleotides: Introducing Modular Tandem Oligonucleotide Synthesis”. Chemistry – An Asian Journal, 2025, e00537.
10. Saraya J. S., Horton N. G., Sammons S. R., O’Flaherty D. K.* “A robust strategy for introducing amino-modifiers in nucleic acids: Enabling novel amino tandem oligonucleotide synthesis in DNA and RNA”. Chemistry – A European Journal, 2025, e202500448 (Inside Cover Art Feature).

9. Saraya J. S., Sammons S. R., O’Flaherty D. K.* “”Aqueous compatible post-synthetic on-column conjugation of nucleic acids using amino-modifiers” ChemBioChem, 2025, 26, e202400643 (invited manuscript) (Cover Art Feature)

8. Robinson J. D., Sammons S. R., O’Flaherty D. K.* “Preparation of 2-aminoimidazole-activated substrates for the study of nonenzymatic genome replication” Current Protocols in Nucleic Acid Chemistry, 2024, 4, e1119
7. Sammons S. R., Robinson J. D., O’Flaherty D. K.* “Efficient synthesis of the 2-aminoimidazolium-bridged diguanosyl intermediate for nonenzymatic primer extension” Canadian Journal of Chemistry, 2024, 102, 706–713 (invited manuscript) (Part of the special issue dedicated to 150 years of chemistry at the University of Guelph)


6. Saraya J. S., O’Flaherty D. K.* “A facile and general tandem oligonucleotide synthesis methodology for DNA and RNA” ChemBioChem, 2024, 25, e202300870 (Very Important Paper) (Front Cover) (Highlighted in ChemistryViews)
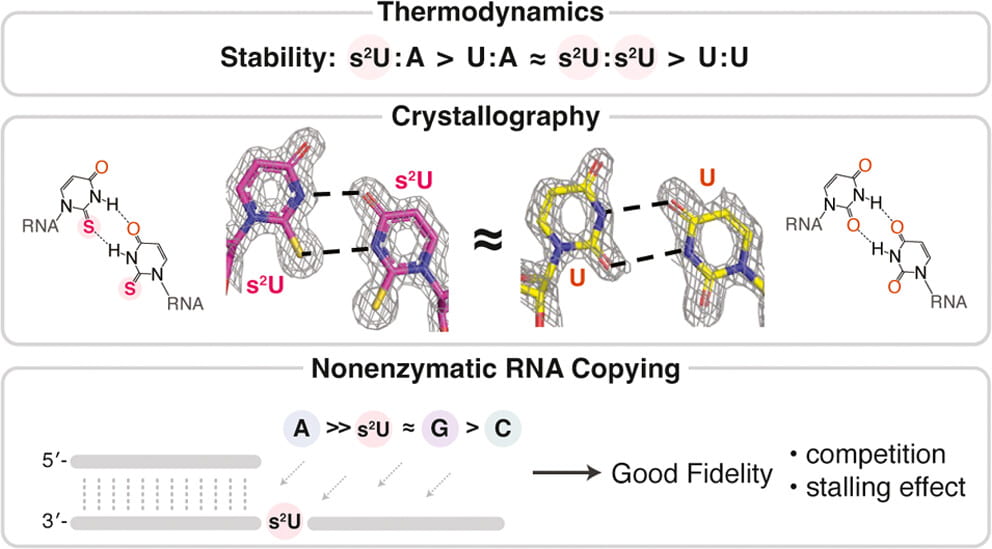
5. Ding D. , Fang Z., Kim S. C., O’Flaherty D. K., Jia X., Stone T. B., Zhou L.*, Szostak J. W.* “An Unusual Base Pair Between Two 2-Thiouridines and its Implication for Nonenzymatic RNA Copying” J. Am. Chem. Soc., 2024, 146, 3861–3871
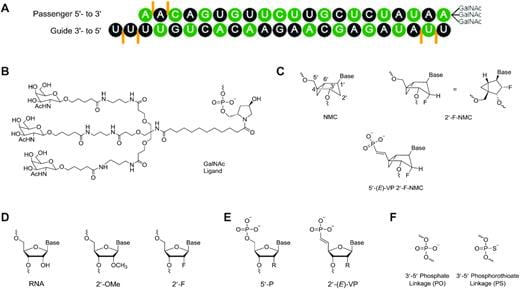
4. Akabane-Nakata M.*, Erande N. D., Kumar P., Degaonkar R., Gilbert J. A., Qin J., Mendez M., Woods L. B., Jiang Y., Janas M. M., O’Flaherty D. K., Zlatev I., Schlegel M. K., Matsuda S., Egli M., Manoharan M.* “siRNAs containing 2′-fluorinated Northern-methanocarbacyclic (2′-F-NMC) nucleotides: in vitro and in vivo RNAi activity and inability of mitochondrial polymerases to incorporate 2′-F-NMC NTPs” Nucleic Acid Res. 2021, 49, 2435–2449.
3. Janicki M., Kufner C. L., Todd Z .R., Kim S. C., O’Flaherty D. K., Szostak J. W., Šponer J., Góra R. W., Sasselov D. S., Szabla R.* “Ribose Alters the Photochemical Properties of the Nucleobase in Thionated Nucleosides” J. Phys. Chem. Lett. 2021, 12, 6707–6713.
2. Rubio-Sánchez R., O’Flaherty D. K., Wang A., Coscia F., Petris G., Di Michele L., Cicuta P., Bonfio C.* “Thermally Driven Membrane Phase Transitions Enable Content Reshuffling in Primitive Cells” J. Am. Chem. Soc., 2021, 143, 16589–16598.
1. Kim S., O’Flaherty D. K., Zhou L., Giurgiu C., Szostak J. W.* “The Emergence of RNA from the Heterogeneous Productsof Prebiotic Nucleotide Synthesis”, J. Am. Chem. Soc., 2021, 143, 3267-3279.
Postdoc and Graduate studies
- ‡ indicates equal contribution; * indicates corresponding authors
29. Zhou, L.‡; O’Flaherty, D. K.‡; Szostak, J. W.* “Assembly of a Ribozyme Ligase from Short Oligomers by Nonenzymatic Ligation”, J. Am. Chem. Soc., 2020, 142, 15961–15965.
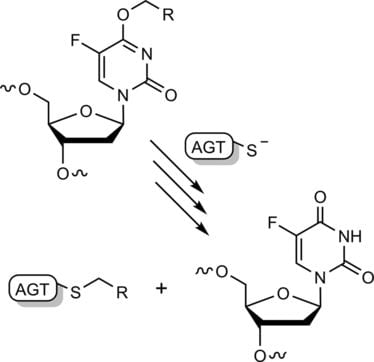
27. O’Flaherty D. K.‡*; Zhou, L.‡; Szostak, J. W.* “Nonenzymatic RNA-templated synthesis of N3′→P5′ phosphoramidate DNA”, Bio-protocol, 2020, 10, e3734.
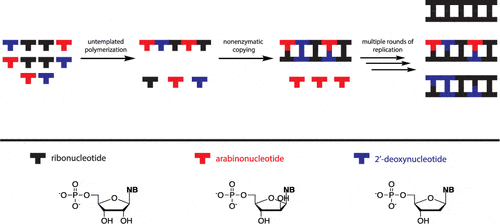
26. Kim S. C.; Zhou L.; Zhang W.; O’Flaherty D. K.; Rondo-Brovettoc V.; Szostak J. W.* “A model for the emergence of RNA from a prebiotically plausible mixture of ribonucleotides, arabinonucleotides and 2′-deoxynucleotides”, J. Am. Chem. Soc., 2020, 142, 2317-2326.
25. O’Flaherty D. K.‡; Zhou L.‡; Szostak J. W.* “Nonenzymatic template-directed synthesis of mixed-sequence 3′-NP-DNA up to 25 nucleotides long inside model protocells”, J. Am. Chem. Soc., 2019, 141, 10481-10488.
24. Zhou L.; Kim S. C.; Ho K. H.; O’Flaherty D. K.; Giurgiu C.; Wright T. W.; Szostak J. W.* “Non-enzymatic primer extension with strand displacement”, eLife, 2019, 8, e51888.
23. Lelyveld V. S.; O’Flaherty D. K.; Zhou L.; Izgu E. C.; Szostak J. W.* “DNA polymerase activity on synthetic N3′–P5′ phosphoramidate DNA templates”, Nucleic Acid Res., 2019, 47, 8941-8949.
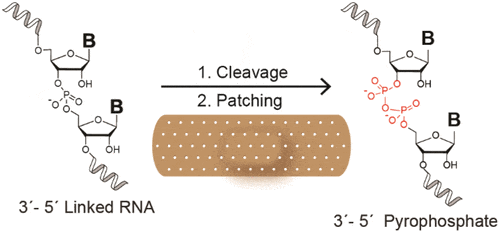
22. Wright T. W.‡; Giurgiu C.‡; Zhang W.; Radakovic A.; O’Flaherty D. K.; Zhou L.; Szostak J. W.* “Prebiotically plausible ‘patching’ of RNA backbone cleavage through a 3′-5′ pyrophosphate linkage”. J. Am. Chem. Soc., 2019, 141, 18104-18112.
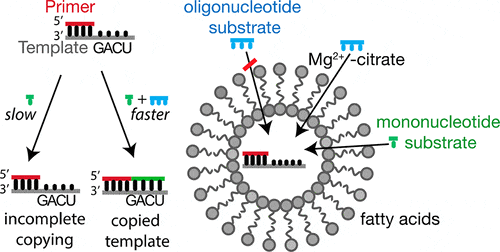
21. O’Flaherty D. K.‡; Kamat N. P.‡*; Mirza F. N.; Li L.; Prywes N.; Szostak J. W.* “Copying of mixed-sequence RNA templates inside model protocells”, J. Am. Chem. Soc., 2018, 140, 5171-5178.
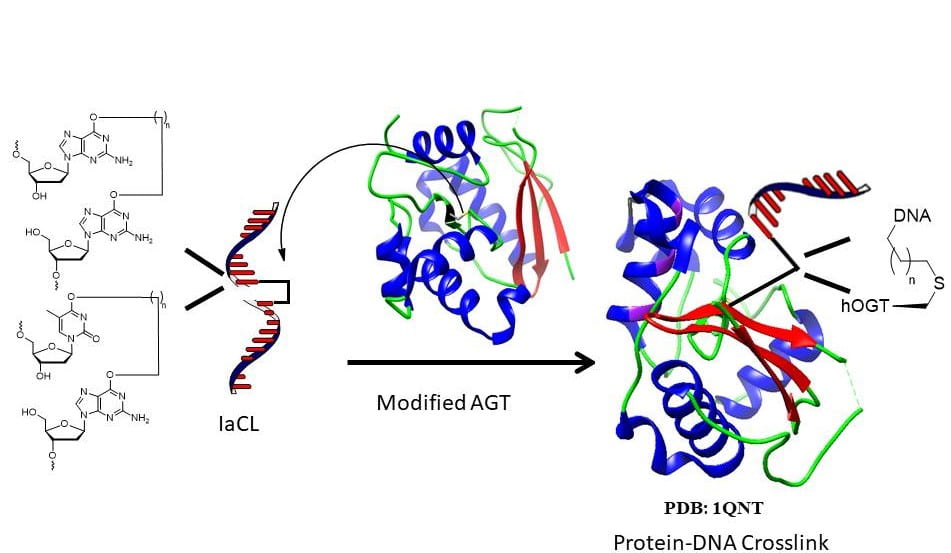
20. Copp W.; O’Flaherty D. K.; Wilds C. J.* “Covalent capture of OGT’s active site using engineered human-E.coli chimera and intrastrand DNA cross-links”, Org. Biomol. Chem., 2018, 16, 9053-9058.
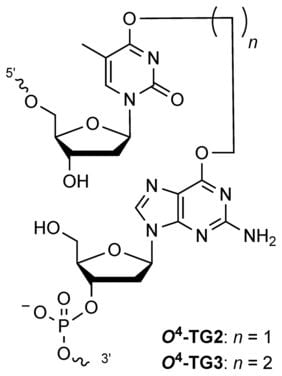
19. Sacre L.; O’Flaherty D. K.; Archambault P.; Copp W.; Peslherbe G. H.; Muchall H. M.; Wilds C. J.*; “O4-alkylated-2- deoxyuridine repair by O6-alkylguanine DNA alkyltransferase is augmented by a C5-fluorine modification. Chembiochem, 2018, 19, 575 – 582.
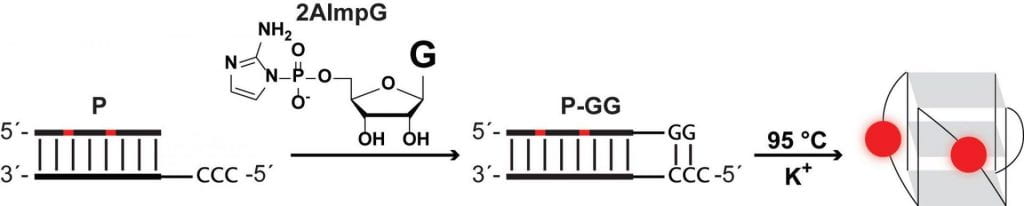
18. Giurgiu C.; Wright T. H.; O’Flaherty D. K.; Szostak J. W.* “A fluorescent G-quadruplex sensor for chemical RNA copying”, Angew. Chem. Int. Ed., 2018 57, 9844-9848.
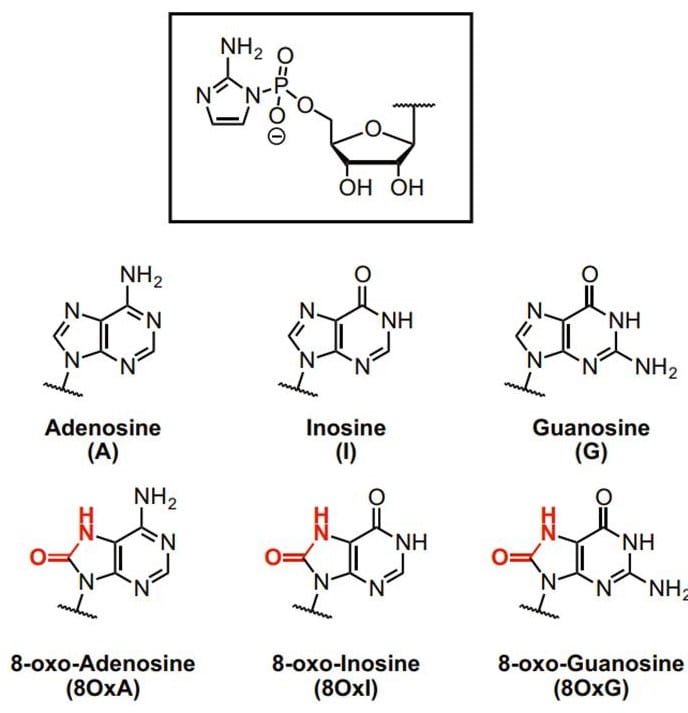
17. Kim S. C.; O’Flaherty D. K; Zhou L.; Lelyveld V. S.; Szostak J. W.* “Inosine, but none of the 8-oxo-purines, is a plausible component of a primordial version of RNA”, Proc. Natl. Acad. Sci. U.S.A., 2018, 115, 13318-13323.
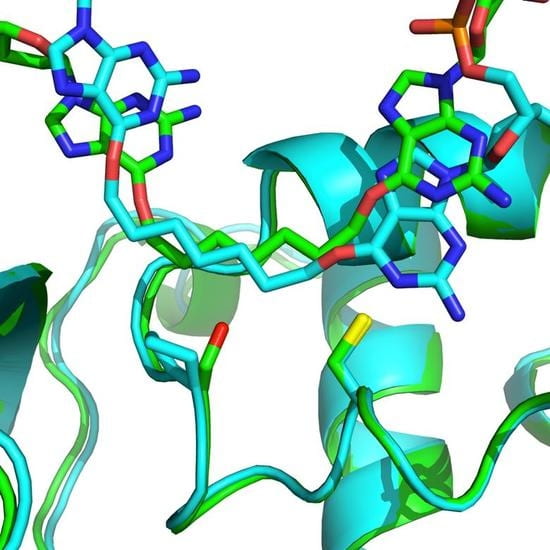
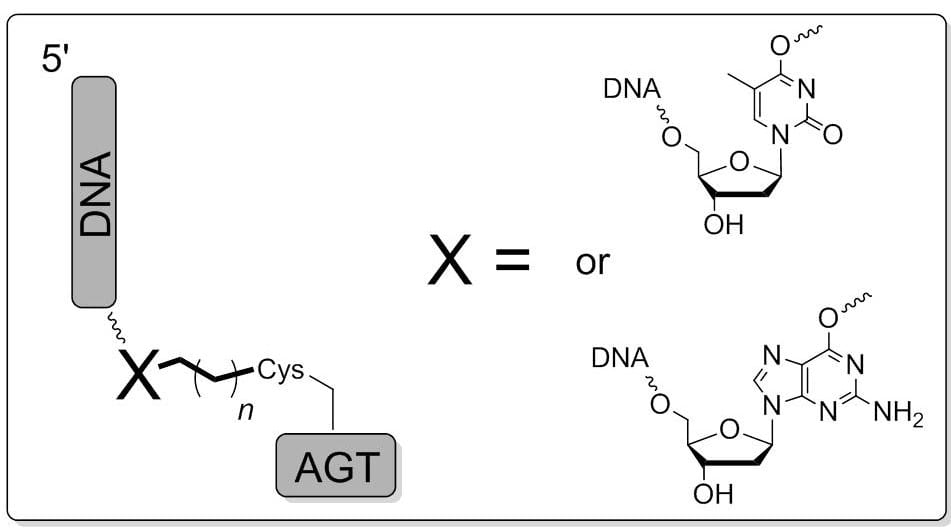
16. O’Flaherty D. K.; Wilds C. J.* “AGT activity towards intrstrand crosslinked DNA is modulated by the alkylene linker”, ChemBioChem, 2017, 18, 2351-2357.
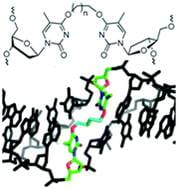
15. Denisov A. Y.‡: McManus F. M.‡; O’Flaherty D. K.; Noronha A. M.; Wilds C. J.* “Structural basis of interstrand cross-link repair by O6-alkylguanine DNA alkyltransferase”, Org. Biomol. Chem., 2017, 15, 8361-8370.
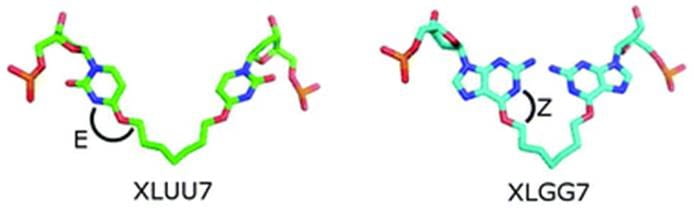
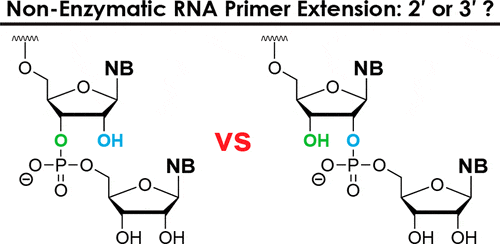
14. Giurgiu C.; Li L.; O’Flaherty D. K.; Tam C. P.; Szostak J. W.* “A mechanistic explanation for the regioselectivity of nonenzymatic RNA primer extension”, J. Am. Chem. Soc., 2017, 139, 16741-16747.
13. Schoonhoven N. M.‡; O’Flaherty D. K.‡; McManus F. P.‡; Sacre L.; Noronha A. M.; Kornblatt M. J.*; Wilds C. J.* “Altering residue 134 confers an increased substrate range of alkylated nucleosides to the E. coli OGT protein” Molecules, 2017, 22, 1948.
12. O’Flaherty D. K.; Wilds C. J.* “Site-specific covalent capture of human O6-alkylguanine-DNAalkyltransferase using single-stranded intrastrand cross-linked DNA”. Org. Biomol. Chem., 2017, 15, 189-196.
11. Li L.; Prywes N.; Tam C. P; O’Flaherty D. K.; Lelyveld V. S.; Izgu E. C.; Pal A.; Szostak J. W.* “Enhanced nonenzymatic RNA copying with 2-aminoimidazole activated nucleotides”, J. Am. Chem. Soc., 2017, 139, 1810-1813.
10. Xu W.; Kool D.; O’Flaherty D. K.; Keating A. M.; Sacre L.; Egli M.; Noronha A. M.; Wilds C. J.; Zhao L.* “O6-2′-Deoxyguanosine-butylene-O6-2′-deoxyguanosine DNA interstrand cross-links are replication-blocking and mutagenic DNA lesions” Chem. Res. Toxicol. 2016, 29, 1872-1882.
9. O’Flaherty D. K.; Wilds C. J.* “Preparation of intrastrand {G}O6-alkylene-O6{G} cross-linked oligonucleotides”, Curr. Protoc. Nucleic Acid Chem., 2016, 66, 5.17.1-5.17.24
8. O’Flaherty D. K.; Wilds C. J.* “O6-Alkylguanine DNA alkyltransferase activity towards intrastrand crosslinked DNA is influenced by the internucleotide linkage”, Chem Asian J, 2016, 11, 576-583.
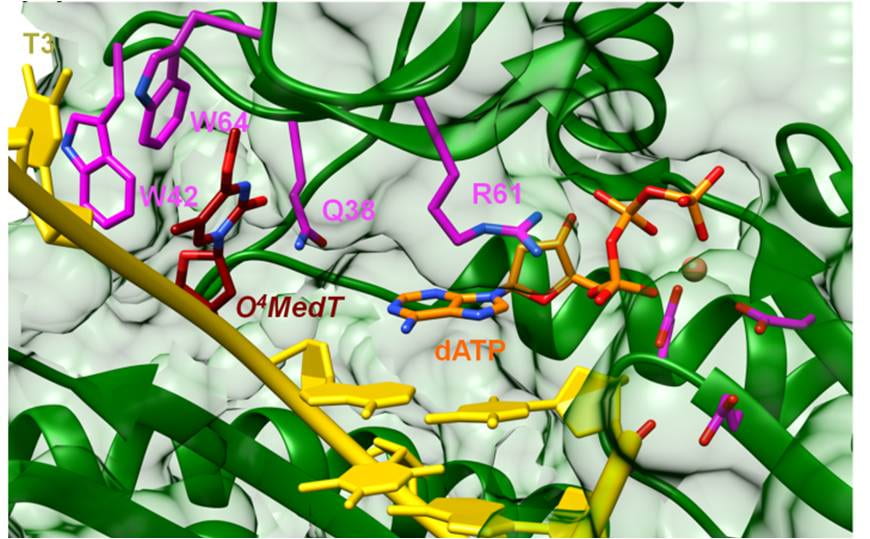
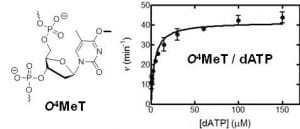
7. O’Flaherty D. K.; Patra A.; Su Y.; Guengerich F. P.; Egli M.*; Wilds C. J.* “Lesion orientation of O4-alkylthymidine influences replication by human DNA polymerase η“, Chemical Science, 2016, 7, 4896-4904.
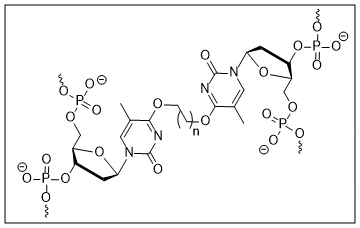
6. O’Flaherty D. K.; Wilds C. J.* “Synthesis, characterization and repair of a flexible O6-2′-deoxyguanosinealkylene-O6-2′-deoxyguanosine intrastrand cross-link”, Chem. Eur. J., 2015, 21, 10522-10529.
5. O’Flaherty D. K.; Guengerich F. P.; Egli M.*; Wilds C. J.* “Backbone flexibility influences nucleotide incorporation by human translesion DNA polymerase eta opposite intrastrand cross-linked DNA”, Biochemistry, 2015, 54, 7449-7456.
4. O’Flaherty D. K.‡; Denisov A. Y.‡; Noronha A. M.; Wilds C. J.* “NMR structure of an ethylene interstrand crosslinked DNA which mimics the lesion formed by 1,3-bis(2-chloroethyl)-1-nitrosourea”, ChemMedChem, 2014, 9
2099-2103.
3. O’Flaherty D. K.; Guengerich F. P.* “Steady-state kinetic analysis of DNA polymerase single-nucleotide incorporation products”, Curr. Protoc. Nucleic Acid Chem., 2014, 59, 7.21.1-7.21.13.
2. O’Flaherty D. K.; McManus F. P.; Noronha A. M.; Wilds C. J.* “Synthesis of Building Blocks and Oligonucleotides Containing {T}O4-Alkylene-O4{T} Interstrand Cross-Links”, Curr. Protoc. Nucleic Acid Chem., 2013, 55, 5.13.1-5.13.19.
1. McManus F. P.; O’Flaherty D. K.; Noronha A. M.; Wilds C. J.* “O4-Alkyl-2′-deoxythymidine cross-linked DNA to probe recognition and repair by O6-alkylguanine DNA alkyltransferases” Org. Biomol. Chem., 2012, 10, 7078-7090.
Published abstracts
1. Schoonhoven N. M.; Murphy S. P.; O’Flaherty D. K.; Noronha A. M.; Kornblatt M. J.; Wilds C. J.* “Synthesis, Biophysical and Repair Studies of O6-2′-deoxyguanosine Adducts by Escherichia coli OGT”, Nucleic Acids Symposium Series, 2008, 52, 449-450